What is corrosion? What types of corrosion are there?
Corrosion is the natural process by which refined metals are transformed into more chemically stable forms such as oxides, hydroxides, carbonates and sulphides. It is the gradual destruction of a material (usually a metal) by a chemical and/or electrochemical reaction with the environment. Rust is the most commonly used word when referring to damaged metal. But rust is not the right name for all metals. Only steel containing iron develops rust. Other types of metal corrode. This can often cause confusion, but corrosion is the all-embracing term. You can read more about it below and see what types there are in the bullets.
- Rusting of steel (iron)
- Stress corrosion
- Oxygen corrosion
- Acid corrosion
- Uniform corrosion
- Stray-flow corrosion
- Galvanic corrosion
- Crevice corrosion
- Pit corrosion
Oxygen corrosion
Oxygen corrosion is the most visible form of corrosion. If, for example, a steel component lies outside in the rain for too long, a reaction will occur. The rain water and humidity cause the steel to corrode. The oxide skin has a brown colour and an open structure. Due to the openness, moisture and oxygen can easily penetrate. The reaction will continue until the steel is finally gone. There are also metal types that have a closed oxide skin of their own, such as: aluminium (ADC12), copper and stainless steel. These metal types offer very good protection against oxygen corrosion.
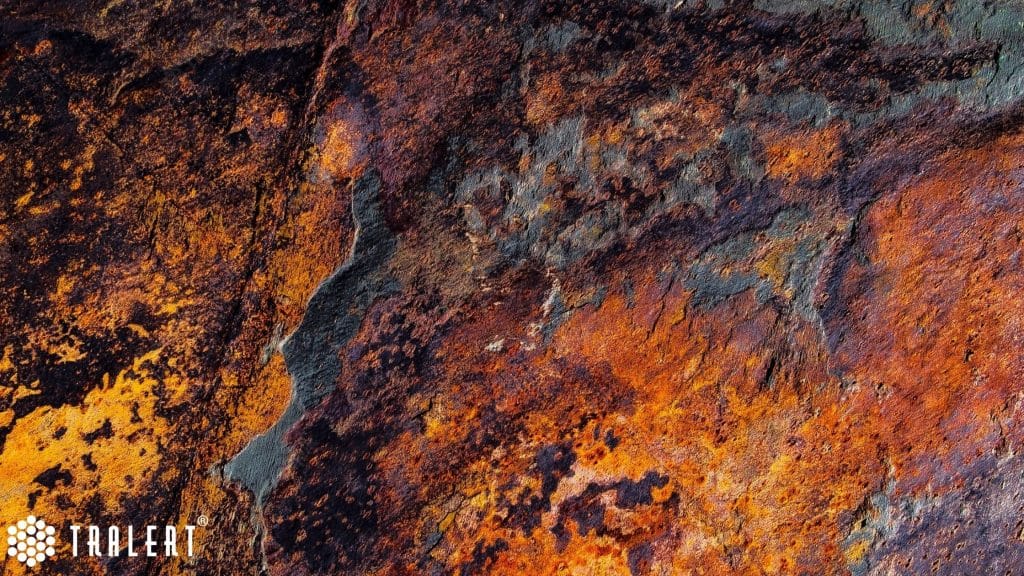
How does rust develop on steel (iron)?
In the case of steel, rust (corrosion) occurs when steel is exposed to oxygen and water. For example, a component made of steel standing outside in the rain. Over time, oxygen and iron combine at an atomic (indivisible) level. This creates a new compound called iron oxide. Water is a good catalyst, which speeds up the whole process. In seawater, this process is much faster because the salt acts as a conductor.
Do all metals corrode?
In principle, all metals can corrode. However, this works slightly differently with precious metals, because these metals are much less reactive than other metal groups. As a result, you hardly have any corrosion. In fact, they are the only metals that can be found in pure form in nature.
The Spartan Series LED Bar from TRALERT® offers the solution!
The Spartan series is equipped with a powerful aluminium housing, as already mentioned, aluminium has a closed oxide skin which offers excellent protection against oxygen corrosion. So you don't have to worry about corrosion! In addition, the compact size is perfect for recessed & surface mounting and the Spartan series is equipped with dual color daytime running lights. The RFT lens techniek ensures efficient and better distributed light distribution. Curious? Take a look at The Spartan LED Bar series.